Calculation of Net Dipole Moment and its Applications
Calculation of Net Dipole Moment and its Applications: Overview
This topic covers concepts, such as, Calculation of Dipole Moment for Symmetrical Molecule, Application of Dipole Moment to Determine Orientation in Benzene Ring & Application of Dipole Moment to Determine Bond Moment etc.
Important Questions on Calculation of Net Dipole Moment and its Applications
Which compounds exhibits maximum dipole moment among the following?
The percent ionic character in is The observed dipole moment is . Find the inter-nuclear distance in .
Give the nearest integer as the answer.
Dipole moment of a bond is a vector and physical quantity to calculate the percentage ionic character in a covalent bond. It is expressed as:
Dipole moment where, is dipole moment and $d$ is the bond length. It is usually expressed in terms of C.G.S. unit known as Debye (D) esu cm. In S.I. unit it is expressed in coulomb metre. Resultant dipole moment of two bond moments acting at an angle , is given by:
(molecule is non-polar)
If $\mu \neq 0$ molecule is polar. Dipole moment plays an important role in deciding the stability order of alkanes,i.e., a more stable alkane has less dipole moment. The dipole moment of a molecule can predict the geometrical and position isomers as well as orientations in benzene nucleus and polarity of molecule.
Dipole moment of molecule is found to be . Assuming bond length to be equal to , the ' ionic character of molecule is:
The dipole moment of 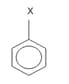 is 1.5 D . The dipole Moment of
is 1.5 D . The dipole Moment of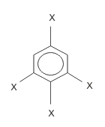 in Debye is
in Debye is
Choose the correct statement from the following
The experimental value of the dipole moment of is . The length of the bond is . The percentage of ionic character in is:
[Given: ]
The net dipole moment of , if the dipole moment of its gauche form is 5D and mole fraction of anti-form is 0.7 is:
Experiment shows that has a dipole moment while has not. Point out the structure which best illustrates these facts
is dipolar, whereas is not. It is because:
The dipole moment of bond and bond is 'X' and 'Y' respectively. If of Debyes, then of will be? Consider the same effect of lone pair on dipole moment of both molecules. (Assuming bond angle of and and
You have been given two species and and two dipole moments and . The correct set is:
Calculate the dipole moment of
If .
You have given two species and and two dipole moments 1.81 D and 0.47 D :
Increasing order of dipole moment is-
Which of the following compounds are non-polar ?
Which of the following compounds has dipole moment approximately equal to that of chlorobenzene?
The dipole moment of is C-m and interatomic spacing is . The % ionic character of HBr is
If the dipole moment of Toluene and Nitro-benzene are D and D respectively, then what is the expected dipole moment of p-Nitrotoluene?
If the molecule of were totally polar, the expected value of dipole moment is (debye). However, the experimental value of dipole moment was . Calculate the percentage ionic character
Which of the following will have a larger dipole moment?

